SURGERY RESEARCH INSIGHT
December 2021
 We Give Back- Vidant Children’s Hospital Wish List Drive
We Give Back- Vidant Children’s Hospital Wish List Drive

Division Updates:
Clinetic to Benefit Surgical Research
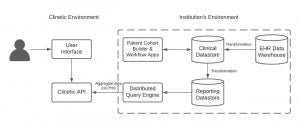
 A collaboration has begun with Clinetic and our department. Clinetic is a health technology company that is able to use the EHR to assist with clinical research. They state in that, “Our software accesses and curates data from electronic health record systems to enable deep insights. Our software can monitor electronic health record data to identify patients who may be eligible for a study, alert a study coordinator, and facilitate collection of additional patient data without an in-person visit.” This software can revolutionize the ability for enrolling clinical research studies. It will cut down on time and make the process of enrollment more efficient. We are currently working to get an agreement in place to be able to move forward with this important collaboration.
A collaboration has begun with Clinetic and our department. Clinetic is a health technology company that is able to use the EHR to assist with clinical research. They state in that, “Our software accesses and curates data from electronic health record systems to enable deep insights. Our software can monitor electronic health record data to identify patients who may be eligible for a study, alert a study coordinator, and facilitate collection of additional patient data without an in-person visit.” This software can revolutionize the ability for enrolling clinical research studies. It will cut down on time and make the process of enrollment more efficient. We are currently working to get an agreement in place to be able to move forward with this important collaboration.
New HCUP Database Acquisition!
-
Pediatric Injury Patterns of North Carolina – investigating risk factors for children resulting from unintentional injuries
-
Improving Rural Surgical Outcomes- evaluating trends in access to surgical care and general surgical outcomes in Eastern NC
-
Has the State of North Carolina Developed an Inclusive Trauma System? – evaluating low and high injury encounters between Trauma and Non-trauma centers, factors associated with the variability rates, and temporal trends over time

Collaboration with the Department of Physiology with Dr. Joseph Houmard (Co-PI), Dr. G. Lynis Dohm (Co-PI), Dr. Walter Pories (Co-I), Dr. Terry Jones (Co-I), Dr. Eric DeMaria (Co-I), and Dr. Nick Broskey (Co-I) and funded by the NIH. This study began July 1, 2019, a five year study with a budget of $2,195,564.
The title of the NIH funded MetFlex study is “Metabolic inflexibility is related to elevated muscle anaerobic glycolysis”. The main focus of the study is on overweight subjects, as these individuals exhibit a high risk of becoming obese and/or developing metabolic diseases. We hypothesize that in some overweight individuals there is a “metabolic program” in skeletal muscle which predisposes them to the development of obesity. A second goal is to investigate if metabolic surgery, which is known to reverse metabolic diseases, changes the muscle metabolic program. Findings from this study may lead to clinical screening tools for determining risk for obesity in non-obese individuals and targeting this group for prevention.
We are currently in year 3 of a 5-year grant and, despite delays due to COVID-19, we have completed studies on approximately 20 overweight subjects. It’s too early to draw any firm conclusions but some of the data is interesting. We are collaborating with faculty and staff in the Department of Surgery to start studies on patients having metabolic surgery.
The Naval Trauma Center Study has recently received IRB approval to begin the task of retrospectively analyzing key clinical outcomes since NMRTC became a Level III trauma center.
The collaboration will continue to strengthen research ties between the Naval Medical Center at Camp Lejeune and the academic research community in Eastern North Carolina.
Study Updates:
Surgical Research Clinical Trials
Currently Enrolling Studies
| Area of Study | Title | Type | Accrual | PI | Contact Info |
|---|---|---|---|---|---|
| Surg Onc | Transportation Barriers to Care in Rural Cancer Patients | Observational | Dr. Drew Honaker | Study Coordinator Kelly Martin martinke21@ecu.edu 744-5723 |
|
| Surg Onc/Pancreas | Trans Intra-arterial Gemcitabine vs. Continuation of IV Gemcitabine plus Nab-Paclitaxel and Radiotherapy for Unresectable Locally Advanced Pancreatic Cancer | Phase II Trial | 11/12 | Dr. Emmanuel Zervos Dr. Andrew Ju | Study Coordinator Denise Brigham brighamd@ecu.edu 744-4924 |
| Surg Onc/Pancreas | Comparing the Clinical Impact of Pancreatic Cyst Surveillance Programs | Interventional | 3/10 | Dr. Emmanuel Zervos Dr. Andrew Ju | Study Coordinator Leslie Corbett corbettl@ecu.edu 744-0456 |
| Surg Onc/Soft Tissue | A Phase III Randomized Trial Comparing Adjuvant MK-3475 (Pembrolizumab) to Standard of Care Observation in Completely Resected Merkel Cell Carcinoma (NCT) | Phase III Trial | 3/2 | Dr. Nasreen Vohra | Study Coordinator Leslie Corbett corbettl@ecu.edu 744-0456 |
| Surg Onc/Soft Tissue | A Phase II Randomized Study of Adjuvant Versus NeoAdjuvant MK-3475 (Pembrolizumab) for Clinically Detectable Stage III-IV High Risk Melanoma | Phase II Trial | Closed to enrollment 5 in follow-up | Dr. Nasreen Vohra | Study Coordinator Leslie Corbett corbettl@ecu.edu 744-0456 |
| Transplant | TRULO - TruGraf Long-term clinical Outcomes | Observational | Closed to enrollment 81 in follow-up | Dr. David Leeser | Study Coordinator Peyton Garris garrisp21@ecu.edu 744-0114 |
| Transplant | ProActive - The PROspera Kidney Transplant ACTIVE Rejection Assessment Registry | Observational | Closed to enrollment 70 in follow-up | Dr. David Leeser | Study Coordinator Peyton Garris garrisp21@ecu.edu 744-0114 |
| Transplant | APOLLO - APOL1 Long-term Kidney Transplantation Outcomes Network | Observational | Closed to enrollment 29 enrolled | Dr. David Leeser | Study Coordinator Peyton Garris garrisp21@ecu.edu 744-5363 |
Operational Updates:
Medical Annex Laboratory 244 (MA 244) Revamp



Dedicated lab and research facilities
The Division of Surgical Research has dedicated facilities and laboratory resources to support our research studies. Medical Annex Suite 244 (MA 244) is a designated space for the Division to conduct research activities and offers space investigators and project staff can use to perform a variety of research study work, from collecting and processing biological specimens to performing non-invasive clinical assessments for research purposes. MA-244 is favorably located in Vidant Medical Center on the second floor and provides our site unique clinical research capabilities.
MA-244 includes five rooms:
Two fully equipped examination rooms

A clinical laboratory space for specimen processing

A storage room that includes a sub-80°C freezer

An office space with four computers reserved for delegated research staff and research residents
A regulatory room designated for compliant research document storage

Research biospecimen processing and storage center
The Division of Surgical Research has sample processing and storage capabilities.
- Equipment currently includes:
- One sub-80°C freezer and specimen refrigerator
- One specimen refrigerator
- A pipetting system for aliquoting of samples
- 1 medium centrifuge
- 4 workstations
- Other general laboratory supplies
2021 Year in Review
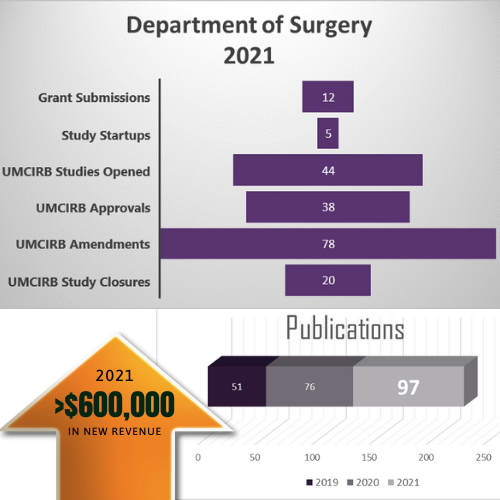
There is many notable 2021 accomplishments for the Department of Surgery. These metrics show the amount of work the research support staff, residents, and faculty have achieved.
Staff Updates:
Medical Students Join Research

Research Residents Projects
So many research projects and such little time!
 Half way into their surgical research year this year’s research residents have been contributing to a widespread of interesting and relevant surgical questions.
Half way into their surgical research year this year’s research residents have been contributing to a widespread of interesting and relevant surgical questions.
 Dr. Josh Aldridge: I am currently working on a project involving ShotSpotter technology in Greenville to identify our firearm injury pattern and determine its effectiveness in reducing gun violence as well as other preventable injuries in our city. I am also involved in a multi-institutional project coordinated through WakeMed identifying the burden of comorbid conditions in young trauma patients.
Dr. Josh Aldridge: I am currently working on a project involving ShotSpotter technology in Greenville to identify our firearm injury pattern and determine its effectiveness in reducing gun violence as well as other preventable injuries in our city. I am also involved in a multi-institutional project coordinated through WakeMed identifying the burden of comorbid conditions in young trauma patients.

Dr. Scarlett Hao: I have been investigating cancer care disparities with Drs. Parikh and Snyder. We’ve found that healthcare reform, specifically the Affordable Care Act and its Medicaid expansion provision, is associated with earlier stage of diagnosis for colon and pancreatic cancers, but that treatment disparities, both racial and insurance-based, persist. I have also led a pilot trial of feasibility utilizing a social determinants of health screening tool built into the Epic EHR for new cancer patient evaluations. We hope to see how identification and intervention on these social determinants can improve cancer care delivery.

Dr. Ashley Quinn: Most of my research focuses on examining the impact social determinants of health have on cancer care delivery. Currently, my main project is examining underlying factors for missed surgical oncology appointments and identify potential areas for intervention. I have also contributed to multiple projects that utilize population-level data, such as the area deprivation index (ADI), to look at health outcomes. I am also involved in quality improvement projects focused on resident wellness and education.

Dr. Seth Quinn: I am currently evaluating trauma induced coagulopathy using platelet mapping thromboelastography (Platelet mapping TEG). We are looking to see if there are certain coagulopathy profiles associated with specific mechanism of trauma (hemorrhage, TBI, long bone fractures, etc.). In the data analysis stage of a multi-institute study looking at the use of cholecystostomy tubes in the treatment of cholecystitis. We are looking for trends in patients and outcomes depending on multiple factors- but primarily the rate of readmissions after placement and outcomes associated with interval between placement and cholecystectomy. I am also involved in multiple quality improvement projects including pre-operative antibiotics and appropriate re-dosing, glycemic control in post-op ERAS patients, ABSITE improvement, and Resident Wellness.
Upcoming Conferences
| Conference | Conference Dates | Location | |||
|---|---|---|---|---|---|
| Safe States | August 20, 2024 | Portland, OR | |||
| NC/SC - American College of Surgeons (ACS)- Annual Meeting | August 23, 2024 | Isle of Palms, SC | |||
| American Association for the Surgery of Trauma (AAST)(TACS) | September 11, 2024 | Las Vegas, NV | |||
| Society of Black Academic Surgeons (SBAS) | September 19, 2024 | Davis, CA | |||
| American Society of Plastic Surgeons (ASPS) | September 26, 2024 | San Diego, CA | |||
| American Academy of Pediatrics (AAP) | September 27, 2024 | Orlando, FL | |||
| CHEST Annual Meeting | October 6, 2024 | Boston, MA | |||
| Association of Women Surgeons (AWS) | October 18, 2024 | San Francisco, CA | |||
| American College of Surgeons (ACS) | October 19, 2024 | San Francisco, CA | |||
| Surgical Outcomes Club (SOC) | October 19, 2024 | San Francisco, CA | |||
| Patient Centered Outcomes Research Institute (PCORI) | October 22, 2024 | Washington, D.C. | |||
| Kidney Week (ASN) | October 23, 2024 | San Diego, CA | |||
| Society of Immunotherapy of Cancer (SITC) | November 6, 2024 | Houston, TX | |||
| ACS TQIP | November 12, 2024 | Denver, CO | |||
| Southern Surgical Association (SSA) | December 8, 2024 | Palm Beach, FL | |||
| National Research Conference for the Prevention of Firearm Related Harms | December 9, 2024 | Seattle, WA | |||
| San Antonio Breast Cancer Symposium | December, 10, 2024 | San Antonio, TX |
Publications
(September 2021-December 2021)
1: Altieri MS. Comment on: Prior bariatric surgery in COVID-19 positive patients
may be protective. Surg Obes Relat Dis. 2021 Sep 4;17(12):e55–6. doi:
10.1016/j.soard.2021.08.026.
2: Altieri MS. Comment on: Impact of a severe complication 2 years after
laparoscopic Roux-en-Y gastric bypass: a cohort study from the Scandinavian
Obesity Surgery Registry. Surg Obes Relat Dis. 2021 Nov;17(11):1882-1883. doi:
10.1016/j.soard.2021.08.009.
3: Tatarian T, Nie L, McPartland C, Brown AM, Yang J, Altieri MS, Spaniolas K,
Docimo S, Pryor AD. Comparative perioperative and 5-year outcomes of robotic and
laparoscopic or open inguinal hernia repair: a study of 153,727 patients in the
state of New York. Surg Endosc. 2021 Dec;35(12):7209-7218. doi:
10.1007/s00464-020-08211-1.
4: Altieri MS, Irish W, Pories WJ, Shah A, DeMaria EJ. Examining the Rates of
Obesity and Bariatric Surgery in the United States. Obes Surg. 2021
Nov;31(11):4754-4760. doi: 10.1007/s11695-021-05628-y.
5: Walsh DS. Proceed, With Caution: Unconscious Bias in Technical Assessment. J
Grad Med Educ. 2021 Oct;13(5):673-674. doi: 10.4300/JGME-D-21-00800.1.
6: Kindel TL, Dirks RC, Collings AT, Scholz S, Abou-Setta AM, Alli VV, Ansari
MT, Awad Z, Broucek J, Campbell A, Cripps MW, Hollands C, Lim R, Quinteros F,
Ritchey K, Whiteside J, Zagol B, Pryor AD, Walsh D, Haggerty S, Stefanidis D.
Guidelines for the performance of minimally invasive splenectomy. Surg Endosc.
2021 Nov;35(11):5877-5888. doi: 10.1007/s00464-021-08741-2.
7: Slater BJ, Dirks RC, McKinley SK, Ansari MT, Kohn GP, Thosani N, Qumseya B,
Billmeier S, Daly S, Crawford C, P Ehlers A, Hollands C, Palazzo F, Rodriguez N,
Train A, Wassenaar E, Walsh D, Pryor AD, Stefanidis D. SAGES guidelines for the
surgical treatment of gastroesophageal reflux (GERD). Surg Endosc. 2021
Sep;35(9):4903-4917. doi: 10.1007/s00464-021-08625-5.
8: Curtis C, Scarcella J, Viscardi C, Samia A, Zeri R, Guo Y. Reduction of
Opioid Prescriptions in Maxillofacial Trauma Following North Carolina STOP Act.
Craniomaxillofac Trauma Reconstr. 2021 Sep;14(3):231-235. doi:
10.1177/1943387520980572.
9: Lim SA, Hao SB, Boyd BA, Mitsakos A, Irish W, Burke AM, Parikh AA, Snyder RA.
Opportunity Costs of Surgical Resection and Perioperative Chemotherapy for
Locoregional Pancreatic Adenocarcinoma. JCO Oncol Pract. 2021 Oct 28:OP2100311.
doi: 10.1200/OP.21.00311.
10: Hermiller JB Jr, Gunnarsson CL, Ryan MP, Moore KA, Clancy SJ, Irish W. The
need for future coronary access following surgical or transcatheter aortic valve
replacement. Catheter Cardiovasc Interv. 2021 Nov 1;98(5):950-956. doi:
10.1002/ccd.29841.
11: Hao S, Snyder RA, Irish W, Parikh AA. Association of race and health
insurance in treatment disparities of colon cancer: A retrospective analysis
utilizing a national population database in the United States. PLoS Med. 2021
Oct 25;18(10):e1003842. doi: 10.1371/journal.pmed.1003842.
12: Lauck SB, Baron SJ, Irish W, Borregaard B, Moore KA, Gunnarsson CL, Clancy S,
Wood DA, Thourani VH, Webb JG, Wijeysundera HC. Temporal Changes in Mortality
After Transcatheter and Surgical Aortic Valve Replacement: Retrospective
Analysis of US Medicare Patients (2012-2019). J Am Heart Assoc. 2021 Oct
19;10(20):e021748. doi: 10.1161/JAHA.120.021748.
13: Barberio MD, Dohm GL, Pories WJ, Gadaleta NA, Houmard JA, Nadler EP, Hubal
MJ. Type 2 Diabetes Modifies Skeletal Muscle Gene Expression Response to Gastric
Bypass Surgery. Front Endocrinol (Lausanne). 2021 Oct 6;12:728593. doi:
10.3389/fendo.2021.728593.
14: Fischer LE, Wolfe BM, Fino N, Elman MR, Flum DR, Mitchell JE, Pomp A, Pories
WJ, Purnell JQ, Patti ME; LABS Investigators. Postbariatric hypoglycemia:
symptom patterns and associated risk factors in the Longitudinal Assessment of
Bariatric Surgery study. Surg Obes Relat Dis. 2021 Oct;17(10):1787-1798. doi:
10.1016/j.soard.2021.04.021.
15: Takeda K, Murray G, Vohra N, Fallon JT. A case of the world’s largest renal
cell carcinoma. IJU Case Rep. 2020 Oct 25;4(1):49-52. doi: 10.1002/iju5.12236.
16: Mitsakos AT, Vohra NA, Fitzgerald TL, Buccini P, Parikh AA, Snyder RA, Zervos
EE. Improvement in Surgical Quality Following Pancreaticoduodenectomy With
Increasing Case Volume in a Rural Hospital. Am Surg. 2021 Nov 18:31348211050808.
doi: 10.1177/00031348211050808.
17: Snyder RA, Parikh AA. Actual Survival in Patients with Resected Pancreatic
Cancer: How Do Real-World Data Compare with Clinical Trial Evidence? Ann Surg
Oncol. 2021 Dec;28(13):8014-8016. doi: 10.1245/s10434-021-10532-x.
18: Snyder RA, Ahmad S, Katz MHG. Pancreas cancer trials for early stage disease:
Surgeons leading therapeutic cooperative group trials. J Surg Oncol. 2021 Sep
29. doi: 10.1002/jso.26701.
19: Boughey JC, Snyder RA, Kantor O, Zheng L, Chawla A, Nguyen TT, Hillman SL,
Hahn OM, Mandrekar SJ, Roland CL. Impact of the COVID-19 Pandemic on Cancer
Clinical Trials. Ann Surg Oncol. 2021 Nov;28(12):7311-7316. doi:
10.1245/s10434-021-10406-2.
20: Chawla A, Nguyen TT, Snyder RA, Boughey JC. ASO Author Reflections: The “New
Normal” in Cancer Clinical Trials in the Post-Pandemic Era. Ann Surg Oncol. 2021
Nov;28(12):7317-7318. doi: 10.1245/s10434-021-10545-6.
21: Snyder RA, He J, Le-Rademacher J, Ou FS, Dodge AB, Zemla TJ, Paskett ED,
Chang GJ, Innocenti F, Blanke C, Lenz HJ, Polite BN, Venook AP. Racial
differences in survival and response to therapy in patients with metastatic
colorectal cancer: A secondary analysis of CALGB/SWOG 80405 (Alliance A151931).
Cancer. 2021 Oct 15;127(20):3801-3808. doi: 10.1002/cncr.33649.
22: Chipman V, Cooper M, Thomas AG, Ronin M, Lee B, Flechner S, Leeser D, Segev
DL, Mandelbrot DA, Lunow-Luke T, Syed S, Hil G, Freise CE, Waterman AD, Roll GR.
Motivations and outcomes of compatible living donor-recipient pairs in paired
exchange. Am J Transplant. 2021 Sep 1. doi: 10.1111/ajt.16821.
23: Garg N, Warnke L, Redfield RR, Miller KM, Cooper M, Roll GR, Chipman V,
Thomas A, Leeser D, Waterman AD, Mandelbrot DA. Discrepant subtyping of blood
type A2 living kidney donors: Missed opportunities in kidney transplantation.
Clin Transplant. 2021 Oct;35(10):e14422. doi: 10.1111/ctr.14422.



