SURGERY RESEARCH INSIGHT
September 2021
TRULO Now Enrolling!
Our site opened enrollment for TRULO July 14, 2021.
On April 15, Eurofins – Transplant Genomics (TGI) announced TRULO; a new multi-center observational study, designed to evaluate post-transplant clinical outcomes in recipients of kidney transplants who are undergoing serial TruGraf® testing.
This will be the first study to provide long-term data, beyond 2 years post-transplant, regarding the benefits of non-invasive surveillance of stable kidney transplant recipients to rule out silent subclinical rejection.
This study is unique in that it focuses on patients that are more remote from their transplant and will correlate TruGraf® and TRACTM monitoring with long-term outcomes. The information from this study has the potential to help improve long term survival of transplanted kidneys.
Our team is eager to begin this multi-institutional study, exploring non-invasive alternatives to detecting transplant rejection!
Learn more about TRULO here TRULO Study to Evaluate Long-Term Clinical Outcomes (trugraf.com)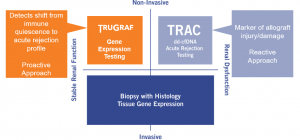
Division Updates:
On July 6, 2021, ASSIST Center headquartered at North Carolina State University formally announced their collaboration with ECU Brody School of Medicine. This collaboration began in September of 2019 with the Department of Surgery and the ASSIST Center. The Division of Surgical Research has submitted two NIH RO1 grants focusing on testing of a wearable biosensing microsystem that extracts, processes, and analyzes dermal biofluids for quantifying longitudinal creatinine and lactate profiles. These multiplex wearables will then be validated in clinical trials involving various surgical populations within our department. The Division of Surgical Research will be submitting two NSF grants this fall as well as another NIH RO1 to continue this collaboration.
ECU Brody School of Medicine and ASSIST Center Partner on Healthcare Access Initiative
Multi-Institutional Trauma Study
Dr. Michelle Brownstein has partnered with the University of North Carolina at Chapel Hill, Duke University, and WakeMed Health and Hospitals in a study titled “Can Trauma be an Opportunity to Identify and Treat Non-Injury Medical Conditions in Young Adults?”. This retrospective study aims to estimate the burden of undiagnosed hypertension, diabetes, and obesity, and unmanaged substance use in young adult trauma patients, to assess the impact of disease on trauma outcomes and healthcare utilization, and to identify patient characteristics associated with undiagnosed disease. The study will examine over 1000 patients from 1/1/2018-12/31/2020. The inclusion criteria include adult patients between the ages of 18 and 40, presented to trauma at Vidant Medical Center, and admitted to the hospital. There are no exclusion criteria.
Study Updates:
Division of Surgical Research Clinical Trials
Currently Enrolling Studies
Kidney Transplantation Studies
TRULO: Co PIs: Dr. David Leeser & William Irish PhD.
- Study Details:
- This is an industry sponsored study that evaluates the impact of monitoring renal transplant patients with both TruGraf® and TRAC™ on long term outcomes in recipients of kidney transplant.
- Subjects who qualify to participate will return to clinic, or a remote clinic, for a total of 9 visits, over the 2-year period.
- Enrollment (Sponsor Goal: 2000 & Site Goal: 100):
- Active: as of July 2021
- Currently Enrolled: All Sites: 474 ECU:7
- Inclusion/Exclusion:
- (age ≥ 18 years)
- kidney transplant patients who are at least one year from the time of transplant.
- Study Coordinator: Peyton Garris: (p) 744-1140, garrisp21@ecu.edu
Proactive: PI: Dr. David Leeser
- Study Details:
- This is an observational research study to better understand how Prospera results are used to manage care of patients after a kidney transplant.
- It will also measure the performance of Prospera testing.
- Data and samples collected including months 1, 2, 3, 4, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33 and 36 post renal transplant, will be used to improve the performance of the Prospera test.
- Enrollment (Sponsor Goal: 4000 Site Goal: 75):
- Active: as of November 2021
- Currently Enrolled: All Sites: 2421 ECU:44
- Inclusion/Exclusion:
- (age ≥ 18 years)
- kidney transplant patients – post transplant up to 2 years.
- Study Coordinator: Peyton Garris: (p) 744-1140, garrisp21@ecu.edu
APOLLO: PI: Dr. David Leeser
- Study Details:
- A NIH sponsored and collaborative study with Wake Forest University that explores the variation in the apolipoprotein L1 gene (called APOL1) and the affect on the kidney.
- The APOLLO Network will test the recipients of kidney transplants and kidney donors for the APOL1 to see how this gene may affect them.
- Enrollment (Sponsor Goal: 3000 Site Goal: NA):
- Active: as of November 2019
- Currently Enrolled: All Sites: ECU:6
- Inclusion:
- Donor and recipient – Self reported African ancestry
- Exclusion:
- Recipient – DNA is not available, donor’s next of kin
- Donor -Biracial or multiracial
- Study Coordinator: Peyton Garris: (p) 744-1140, garrisp21@ecu.edu
Surgical Oncology Studies
Transportation Barriers to Care in Rural Cancer Patients: PI: Dr. Rebecca A. Snyder
- Study Details: Access to Cancer Care for Rural Patients: Developing and Implementing a Validated Measure to Identify Patients at High Risk of Transportation Barriers
- Enrollment:
- Active: as of 02-Jan-2020
- Currently Enrolled: 79
- Inclusion/Exclusion:
- Patients considered eligible for the study must meet the following study criteria: 1) adult patients (age 18 years) 2) living in a rural residence defined at the county-level by the Federal Office of Rural Health Policy with 3) newly diagnosed colorectal, pancreas, breast, or non-small cell lung cancer of any American Joint Commission on Cancer (AJCC) stage who are scheduled for evaluation or treatment at Vidant Medical Center (VMC). Patients will be excluded if they reside in a non-rural (i.e. metropolitan) county.
- Study Coordinator: Tiffanie Moore
ATOMIC : PI: Dr. Rebecca A. Snyder
- Study Details:
- Randomized Trial of Standard Chemotherapy Alone Or Combined With Atezolizumab As Adjuvant Therapy For Patients With Stage III Colon Cancer And Deficient DNA Mismatch Repair
- Enrollment:
- Active: as of 10/25/2019
- Currently Enrolled: 0
- Inclusion:
- Resected stage III colon adenocarcinoma (any T, N1-2M0; includes N1C).
- Must have deficient (d) DNA mismatch repair (dMMR).
- MMR status must be assessed by immunohistochemistry (IHC) for MMR protein expression (MLH1, MSH2, MSH6, PMS2) where loss of one or more proteins indicates dMMR.
- Study Coordinator: Amanda Brunni: (p) 744-5687
EA2185 PI: Dr. Rebecca A. Snyder
- Study Details:
- Comparing the Clinical Impact of Pancreatic Cyst Surveillance Programs
- Enrollment:
- Active: as of 8/1/2020
- Currently Enrolled: 2
- Inclusion:
- Patient must be ≥ 50 years and ≤ 75 years of age.
- Patient must not have acute pancreatitis or a history of chronic pancreatitis.
- Patient must have received a CT, MRI, or EUS within 6 months prior to randomization that revealed one or more ≥ 1 cm pancreatic cyst (s)
- Study Coordinator: Leslie Corbett: (p) 744-0456
Central Pancreatic Consortium: PI: Dr. Rebecca A. Snyder
- Study Details:
- Survey of Patient Understanding of Pancreatic Cancer Resection
- Enrollment:
- Active: as of 4/16/2021
- Currently Enrolled: 0
- Inclusion:
- All patients undergoing pancreatectomy for adenocarcinoma.
- Study Coordinator: nursing staff
Operational Updates:
Regulatory
COVID-19 Research Update
On August 18, 2021, the Office of Clinical Trials updated Brody School of Medicine on the requirements for continuing research during COVID-19. Currently all new research is on pause due to the Center for Research and Grants Office at Vidant’s role in re-allocation due to the increase in COVID-19 cases. This “pause” is expected to continue until the cases of COVID-19 decrease. All human subject research studies are which have study participants are required to complete the following:
- All study team staff must complete COVID-19 Back to Campus Fall 2020 CITI Module
- COVID-19 Active Screening Form
- COVID-19 Safety Plan
- COVID-19 Risk Assessment Plan
- If the Risk Assessment Plan is Low or Medium Risk, the plan must be submitted to Dr. J. Elizabeth Tuttle, Department of Surgery Chair and the Dr. Russ Price, Associate Dean of Research, Brody School of Medicine.
- If the Risk Assessment is deemed High or Very High Risk, the plan must be submitted to the Human Subject Research Advisory Board for approval. This committee meets bi-weekly to review and approve these plans.
- For more information on what information is required for these forms, review the slide presentation:
Grant Update
Reba Bullard presented the Grant Empowerment Workshop in September. This workshop gave an overview of the grant submission process at ECU including eTRACS basics.

Resident and Student Updates

Dr. Helen Johnson nominated for the Jameson L. Chassin, MD, FACS, Award for Professionalism in General Surgery.
To recognize Jameson L. Chassin, MD, FACS, and his career and professional interests, the ninth annual award for chief residents is being offered by the Chassin family, contributors to the Chassin Memorial Fund, and the American College of Surgeons Foundation. In accordance with the wishes of the Chassin family, the Jameson L. Chassin, MD, FACS, Award for Professionalism in General Surgery will be presented to a chief resident in general surgery who exemplifies the values of compassion, technical skill, and devotion to science and learning. Helen Johnson, MD has been nominated for this prestigious award with her history of clinical skills, passion for research, and sincere empathy for her patients.

Dr. Anastasios Mitsakos nominated for ACS Resident Award for Exemplary Teaching
The American College of Surgeons (ACS) Resident Award for Exemplary Teaching, sponsored by the ACS Division of Education, is given each year in recognition of excellence in teaching by a resident and to emphasize teaching as an important area in residents’ daily lives. Anastasios Mitsakos has been nominated for this award with his superb teaching abilities both in the operating room and through his research in the Clinical Simulation Lab.

Dr. Caitlin Pipkin recipient of the 2021 Eastern Cardiothoracic David Campbell Scholarship Dr. Caitlin Takahashi was awarded the David Campbell Cardiothoracic Surgery Scholarship for Residents by the Eastern Cardiothoracic Surgical Society ECTSS. Residents applied not just nationally but internationally. This scholarship will help her attend the 59th annual ECTSS meeting in October 2021, other activities on site, network etc. We are proud of her and the work she is doing.


Dr. William Irish and Dr. Walter Pories mentored four Brody School of Medicine Summer Scholars Research Program participants.
Rufus Aderounmu, Joshua Keku, Virginia Vasquez-Rios, and Mizuki Suzuki participated in the Summer Scholars program with Drs. Pories and Irish as their mentors. Rufus, Josh, and Virginia worked with Dr. Pories on three projects involving bariatric surgery and the metabolic syndrome. Dr. Irish mentored Mizuki Suzuki on a project involving UNOS Kidney Transplantation data to evaluate the effect of HLA mismatch on graft survival post-kidney transplantation in the pre-KAS versus post-KAS allocation era.
